5fr Feeding Tube as Umbilical Catheter
Iowa Neonatology Fellows (including Jon E. Mazursky, MD, Chetan A. Patel, MD, Mark W. Thompson, MD) and John Dagle, MD, PhD
Peer Review Status: Internally Peer Reviewed
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
Purpose
To differentiate fetal blood from swallowed maternal blood in the evaluation of bloody stools.
Method
Mix specimen with 3-5 ml of tap water and centrifuge. Supernatant must have a pink color to proceed. To 5 parts of supernatant, add 1 part of 0.25 N (1%) NaOH.
Interpretation
A pink color persisting over 2 minutes indicates fetal hemoglobin. Adult hemoglobin gives a pink color that becomes yellowish brown in 2 minutes or less indicating denaturation of the adult hemoglobin.
Iowa Neonatology Fellows and John Dagle, MD, PhD
Peer Review Status: Internally Peer Reviewed
The controversial indications for circumcision are thoroughly discussed in the literature and are dealt with in the AAP policy statement, Pediatrics Vol. 130 No. 3 September 1, 2012, pp. 585 -586.
Abstract
Male circumcision is a common procedure, generally performed during the newborn period in the United States. In 2007, the American Academy of Pediatrics (AAP) formed a multidisciplinary task force of AAP members and other stakeholders to evaluate the recent evidence on male circumcision and update the Academy's 1999 recommendations in this area. Evaluation of current evidence indicates that the health benefits of newborn male circumcision outweigh the risks and that the procedure's benefits justify access to this procedure for families who choose it. Specific benefits identified included prevention of urinary tract infections, penile cancer, and transmission of some sexually transmitted infections, including HIV. The American College of Obstetricians and Gynecologists has endorsed this statement.
Policy statement
Systematic evaluation of English-language peer-reviewed literature from 1995 through 2010 indicates that preventive health benefits of elective circumcision of male newborns outweigh the risks of the procedure. Benefits include significant reductions in the risk of urinary tract infection in the first year of life and, subsequently, in the risk of heterosexual acquisition of HIV and the transmission of other sexually transmitted infections.
The procedure is well tolerated when performed by trained professionals under sterile conditions with appropriate pain management. Complications are infrequent; most are minor, and severe complications are rare. Male circumcision performed during the newborn period has considerably lower complication rates than when performed later in life.
Although health benefits are not great enough to recommend routine circumcision for all male newborns, the benefits of circumcision are sufficient to justify access to this procedure for families choosing it and to warrant third-party payment for circumcision of male newborns. It is important that clinicians routinely inform parents of the health benefits and risks of male newborn circumcision in an unbiased and accurate manner.
Parents ultimately should decide whether circumcision is in the best interests of their male child. They will need to weigh medical information in the context of their own religious, ethical, and cultural beliefs and practices. The medical benefits alone may not outweigh these other considerations for individual families.
Findings from the systematic evaluation are available in an accompanying 32-page technical report-
Male Circumcision Task Force Statement. The American College of Obstetricians and Gynecologists has endorsed the above Task Force statement.
FAQ
What are the potential advantages of newborn circumcision?
The glans of the penis is easier to keep clean if the male is circumcised. Because of this ease of cleanliness, certain infections which can occur in uncircumcised boys or men with poor hygiene cannot occur in those who are circumcised. Circumcision will prevent several conditions that cause an accumulation of fluid and swelling around the foreskin and glans, as well as a problem known as phimosis, which is the inability to retract the foreskin. Newborn circumcision protects against the later development of cancer of the penis, although this is an extremely rare disease. Also the incidence of urinary tract infection in male infants is decreased when circumcision is performed during the newborn period.
What are the potential risks and disadvantages of circumcision?
The immediate risks of circumcision are bleeding, inadvertent injury to the remainder of the penis, and infection. Although circumcision is considered to be a generally safe procedure, in rare cases these or other complications can lead to severe problems and even death. Inflammation of the external urethral opening (meatitis) is more common in circumcised boys. Newborn circumcision is usually performed without anesthesia. Although the procedure is relatively brief, the newborn experiences some pain and discomfort.
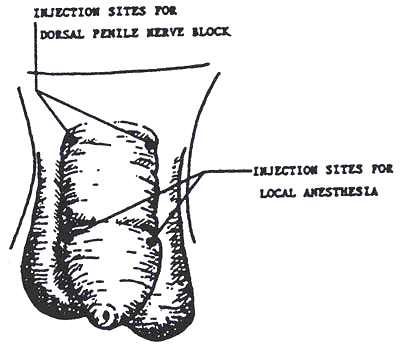
What about local anesthesia?
In recent years, there has been more interest in providing local anesthesia for this procedure, but by no means is this universally accepted. Local anesthesia is provided by injecting a medication into the nerves at the base of the penis. If performed properly, this procedure will reduce the infant's pain and behavioral changes. Complications due to local anesthesia are rare and consist mainly of bleeding and damage to the skin where the injection occurs. Local anesthesia adds an additional element of risk to the procedure.
Method
The pediatric chief residents are given instruction in Gomco clamp circumcision and dorsal penile block. The Gomco clamp method is described in detail in the chapter entitled "Circumcision" 378-388 in Atlas of Procedures in Neonatology, 2nd edition. Fletcher and MacDonald (eds), 1993, JB Lippincott Co., Philadelphia.
Iowa Neonatology Fellows
Revised John Dagle MD, PhD
Peer Review Status: Internally Peer Reviewed
Any infant, especially those born preterm, receiving greater than ambient oxygen concentration must have his arterial oxygen tension or saturation monitored.
An ill infant without an indwelling arterial catheter should have arterial O2 tension monitored by arterial puncture or transcutaneous PO2 monitor. An acceptable alternative would be continuous pulse oximetry with the upper saturation alarm limit set at 95%, but caution should always be used to prevent exposure to high amounts of oxygen. If questions arise regarding the appropriate level of oxygen saturation, peripheral arterial puncture should be performed.
Frequency of sampling depends on the clinical situation and the reliability of the other monitoring devices. Generally, a significant change in ventilator or CPAP setting should be followed by a capillary or arterial sample within 15 minutes to an hour. If performing a peripheral arterial puncture for blood gas purposes, note should be made of the location, as many infants have shunting through the ductus arteriosus that may affect the interpretation.
The amount of blood needed for laboratory tests with peripheral arterial puncture should be determined prior to puncture. The syringes used for blood gas sampling can be obtained from the blood gas laboratory.
Arterial puncture, although not as commonly used in NICU's as other methods of monitoring, can be performed with relative ease, using the radial, temporal, posterior tibial, or dorsalis pedis artery. The brachial and femoral artery should be used only in emergency situations, because of the risk of complications at those sites. Indwelling catheters may be placed in the radial, posterior tibial or dorsalis pedis artery but should not be placed in the temporal or brachial artery.
Prep the site with 3 alcohol swabs and wear appropriately fitting gloves. Goggles or eyeglasses are also recommended. The artery should be easily palpable or visible with transillumination. If using the radial artery, an Allen test should be performed prior to puncture. An arm board may be useful to prevent extreme dorsiflexion of the wrist which makes the procedure more difficult. A 25 gauge butterfly needle, with TB or 3 ml syringe should be used. The bevel up position should be used, except in the most superficial arteries. The angle of insertion should be 25o for a superficial and 45o for a deep artery, against the flow of the artery. Blood should flow spontaneously or with gentle suction.
After the needle is removed, continuous pressure should be applied for 5 minutes, with care not to squeeze with the fingertips. If hematoma formation is prevented, the artery may be used multiple times. Observe the extremity for 15-20 minutes after the procedure for arterial spasm.
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
If an exchange transfusion is necessary, compatible blood must be ordered. If a severely affected ( i.e. hydropic) infant with Rh hemolytic disease is anticipated at birth, it may be necessary to have blood available in the nursery prior to the delivery. The request should be for O negative packed red blood cells of the specific volume needed and of the appropriate CMV status. This blood may be utilized in any one of the following ways:
- The RBC's may be given as a simple transfusion (with or without additional Plasmanate) while stabilization of the infant is accomplished.
- The RBC's may be used for a partial exchange transfusion to acutely elevate the hematocrit without changing the blood volume in a severely anemic baby.
When the need for an emergency, complete exchange transfusion is virtually certain, arrangements can be made in advance for O negative whole blood or O negative PRBC's resuspended in fresh frozen plasma.
For double-volume exchange transfusions for hemolytic disease of the newborn or for hyperbilirubinemia without hemolysis, the blood used will be packed cells (type O, Rh specific for the infant) resuspended to the desired hematocrit in compatible fresh frozen plasma.
A partial exchange transfusion is often done for polycythemia (see section on polycythemia).
Although the standard anticoagulant (CPD) is acidic, the blood need not be buffered. If the infant is severely acidemic, consult the staff neonatologist.
If possible, the infant should be NPO and the stomach contents aspirated prior to the procedure.
The exchange transfusion should be done under a radiant warmer using sterile technique.
The donor blood should be warmed using the blood warmer to a temperature not exceeding 37oC.
The infants blood pressure, respiratory rate, heart rate and general condition should be monitored during the exchange transfusion according to standard nursing protocol.
If the serum bilirubin concentration is at a dangerous level and the blood for exchange transfusion is not yet ready, consider priming the infant with 1 gram/kg (4 ml/kg) of a 25% solution of salt-poor albumin to bind additional bilirubin and keep it in the circulation until the exchange can be accomplished..
The umbilical vein catheter should be inserted until there is free flow of blood immediately prior to starting the exchange transfusion. See section on placement of umbilical catheters for technique. The exchange transfusion should not be done through an umbilical artery line unless the UAC is used only for blood withdrawal with simultaneous replacement through the umbilical vein or peripheral IV. At the beginning of the exchange transfusion, the first blood sample withdrawn should be sent for for 1)total and direct bilirubin; 2) hemoglobin and hematocrit; 3) glucose; and 4) calcium.
Use the "exchange transfusion kit", which contains catheters, stopcocks, waste bag, and calcium gluconate.
Ideally, blood (or colloid in the event of a partial volume exchange) should be infused through a peripheral vein at a rate equal to blood withdrawal from the UVC. If the "push-pull" (single catheter) technique is utilized, no more than 5 ml/kg body weight should be withdrawn at any one time.
The exchange volume is generally twice the infant's blood volume, (generally estimated to be 80 ml/kg). The total volume exchange should not exceed one adult unit of blood (450-500 ml). A standard two-volume exchange will remove approximately 85% of the red cells in circulation before the exchange and reduce the serum indirect bilirubin level by one-half. The exchange of blood should require a minimum of 45 minutes.
The need for giving supplemental calcium is controversial. If used give 0.5 to 1.0 ml of 10% calcium gluconate IV, after each 100 ml of exchange blood. Monitor heart rate for bradycardia.
At the end of an exchange transfusion blood should be sent for sodium, glucose, calcium, total and direct bilirubin, and hemoglobin and hematocrit.
At the end of an exchange transfusion, the umbilical vein catheter is usually removed. In the event of a subsequent exchange, a new catheter can be inserted.
Hypoglycemia often occurs in the first or second hour following an exchange transfusion. It is therefore necessary to monitor blood glucose levels for the first several hours after exchange.
The serum bilirubin concentration rebounds to a value approximately halfway between the pre- and post- exchange levels by two hours after completing the exchange transfusion. Therefore, the serum bilirubin concentration should be monitored at two to four hours after exchange and subsequently every three to four hours.
Feedings may be attempted two to four hours after the exchange transfusion.
Iowa Neonatology Fellows
Revised John Dagle MD, PhD
Peer Review Status: Internally Peer Reviewed
Umbilical line placement
- Umbilical artery catheters (UAC) are used primarily for monitoring blood pressure and obtaining samples for blood gases. In order to maintain the patency of the catheter, a saline solution containing heparin (0.25 U/ml) is infused through the line. Medications and other solutions, including parenteral nutrition solutions, should be given through a venous line (peripheral or central), unless discussed with the staff neonatologist.
- Umbilical vein catheters (UVC), are used for exchange transfusions, monitoring of central venous pressure, and infusion of fluids (when passed through the ductus venosus and near the right atrium); and for emergency vascular access for infusions of fluid, blood products or medications.
- Before the procedure is begun, the correct depth of the umbilical artery catheter insertion should be estimated (see #6 below). Sterile gowns and gloves should be worn, as well as a head cover and a mask. A sterilized umbilical catheterization tray with the necessary instruments and drapes is available in the nursery. After opening the tray, alcohol and sterile syringes, stopcocks, catheters and saline will be placed on it. Sterile technique must be observed; the use of goggles (or eyeglasses) is recommended.
- An umbilical catheter with a single end hole may be used for the catheterization of either umbilical artery or vein. On most occasions, it is advantageous to place a double lumen UVC. Infants with a birth weight of less than 1.5 kg will usually require a 3.5-Fr catheter for arterial catheterization. 5-Fr catheters are used for arterial placement in larger infants. In general, umbilical venous catheters are 5 Fr. For babies less than 500 grams, 3.5 Fr double lumen catheters are available.
- A sterile stopcock is attached to all ports on all lines and the system is flushed with saline to remove all air bubbles.
- External measurements are made to determine how far the catheter should be inserted. In a high setting, the arterial catheter tip (UAC) should be positioned between the sixth and tenth thoracic vertebrae on chest x-ray. This can be achieved by inserting the catheter 1 cm more than the infant's umbilical-to-shoulder length or 2 cm more for term infants or 9 cm + (3 cm x wt in kg). This placement is traditionally preferred. A low-lying arterial catheter should have the tip at the third to fourth lumbar vertebra calculated at 2/3 the infant's umbilical-to-shoulder length.
- The infant's abdomen and cord are cleaned with alcohol. The alcohol should be sparingly applied to prevent pooling under the infant's back and buttocks. In infants less than 1.5 kg, only the cord is cleaned to avoid chemical injury to the abdominal skin. The area is then draped so that only the cord is exposed.
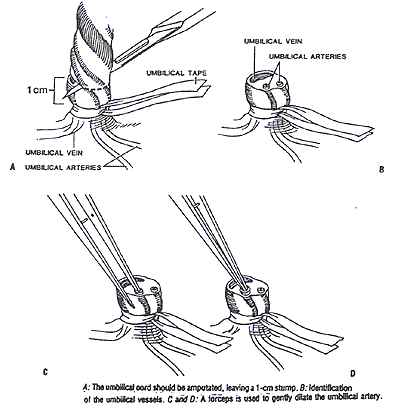
- Tie a piece of umbilical tape around the base of the umbilical cord tightly enough to minimize blood loss but loosely enough so that the catheter can be passed easily through the vessel.
- Using a scalpel, the cord is cut cleanly 1.0 cm from the skin. An alternative method is to hold the cord with a hemostat and gently twist back to expose the cord. A scalpel is used to cut about 2/3 of the way through the cord.
- The cord is stabilized with a forceps or hemostat, and the vessels identified. The single, large, thin walled oval vein can readily be distinguished from the two smaller, thick-walled round arteries (see diagram). If the cord is cut close to the skin the 2 arteries tend to be located more caudally, while the large single vein tends to be located more cephalad.
- The arteries are usually constricted, so that the lumens appear pin-point in size. By gently inserting the closed tips of the curved iris forceps into the lumen of the artery until the cut end of the artery is at the bend in the forceps, and then allowing the spring of the forceps to gently spread the tips, the artery can be carefully dilated.
- Grasping the catheter with a forceps or between the thumb and forefinger, the catheter can be inserted into the lumen of the dilated artery. Supporting the stump is usually necessary. Once the catheter has been inserted, it may encounter resistance at the level of the anterior abdominal wall or at the bladder. This resistance can usually be overcome by application of gentle, steady pressure. Repeated probing movements or excessive pressure must be avoided to prevent pushing the catheter outside of the vessel lumen (false tracking). If unsuccessful, wait 2-3 minutes until the vasospasm ceases, or attempt the other umbilical artery.
- After the catheter is advanced the appropriate distance, the position of the catheter should be confirmed by x-ray.
- Observe both legs for evidence of blanching, cyanosis or mottling. If a "blue leg" develops (presumably from vasospasm), the catheter should be removed or carefully observed for a short period of time to allow for resolution of the impaired circulation.
- After placement of the catheter, a purse-string suture is placed around the umbilicus taking care not to puncture the catheter.
- The procedure for catheterization of the umbilical vein (UVC) is similar; differences are as follows:
- Remove any visible clots from the lumen of the vein with forceps.
- Never leave the catheter open to atmospheric pressure. The abdominal venous system is under negative pressure; with a deep inspiration air can enter the catheter with resultant air embolism.
- For administration of fluid, the venous catheter must be in the inferior vena cava, just below the right atrium. Inserting the catheter two-thirds of the shoulder-to-umbilicus distance is a good estimate. A catheter in the portal venous system must not be used for the long-term administration of fluids or medications and should be removed once better central access can be obtained.
- For the purpose of an exchange transfusion, the catheter should be advanced only until there is a free flow of blood, but never more the 8 cm in the full term infant. This catheter should be used only for withdrawal of blood (see section on Exchange Transfusion).
- If code medications and/or fluid need to be given in the delivery room, a UVC should be placed and advanced only until there is a free flow of blood as in "D" above.
- To sample blood from an umbilical catheter, withdraw 1 ml of blood into a sterile syringe, keeping the syringe perpendicular to the infant. This will cause the blood to settle near the tip of the syringe. The tip of the syringe should be kept sterile, and not placed in the infant's incubator or bed.
- The blood sample is then withdrawn into a second syringe and the initially withdrawn blood reinfused and the system flushed with a small amount of saline until free of blood.
- The alcohol should be washed off with sterile water after the procedure is completed. This is important to prevent clinical burns, especially in very small infants.
- The umbilical artery catheter is removed slowly (~1cm/minute after withdrawing to 6 cm) when it is no longer needed. With proper care, the catheter need not be changed for the duration of its use.
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
Indication
In critically ill infants, placement of intravenous catheters is often difficult and time consuming. The intraosseous route offers immediate vascular access required for emergency administration of drugs during resuscitation. Intraosseous infusion uses the rich vascular network of long bones to transport fluids and drugs from the medullary cavity to the circulation. The response and distribution of fluid and drugs injected via the intraosseous route appears to be very similar to that after intravenous injection. The procedure should be limited to emergencies in which intravenous access (including umbilical vein catheterization) cannot be established in a reasonable length of time, usually 2-5 minutes.
Method
- Insertion of a needle into the medullary cavity of a long bone should be rapid and simple.
- In infants less than 12 months of age, a 16- or 18-gauge spinal needle with a stylet is recommended.
- The preferred site is the medial proximal tibia because of its broad flat surface and thin layer of skin covering the bone.
- A point is selected 1 to 2 cm below the tibial tuberosity on the medial flat surface of the anterior tibia.
- The needle is directed at an angle of 60 degrees pointing away from the joint space and growth plate with a screwing motion.
- Entry into the marrow space is noted by a decrease in resistance. The distance from the skin through the bony cortex is rarely more the 1 cm. A common mistake is to advance the needle into or through the opposite side of the bone.
- To confirm placement, a saline filled syringe is attached to the catheter and infused slowly while palpating the limb for extravasation.
- Drugs may be administered rapidly or by slow infusion.
- Conventional vascular access should be established with discontinuation of the intraosseous infusion as soon as reasonably possible.
Complications
- Success rate is about 80%.
- The most common complication is subcutaneous or subperiosteal infiltration of fluid.
- Risks of cellulitis and osteomyelitis are less than 1% and related to duration of catheter placement.
- No lasting negative effects on growth plate development have been reported.
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
Lumbar puncture (LP) should be performed for the following indications
- Diagnosing or ruling out sepsis in the neonatal period. Meningitis may be present in as many as 25% of cases of neonatal sepsis. The choice and dose of antibiotics and the duration of antibiotic therapy may be longer for patients with meningitis than with sepsis.
- To monitor efficacy of antibiotic therapy with repeat cell count and culture, or occasionally drug levels of antibiotics.
- LP is sometimes used as a treatment for communicating hydrocephalus.
Possible contraindications
- Severe bleeding diathesis
- Superficial infection at the LP site
- Vertebral anomalies
- Increased ICP with decreased communication of spinal fluid
- Severe cardiorespiratory instability of patient
Technique
- Have assistant restrain patient in either lateral decubitus or sitting position with spine flexed. The head need not be flexed too far, as some infants develop increased respiratory difficulty with flexing of the head. Some people prefer the lateral decubitus for the more unstable patients. Complete immobilization of the spine is extremely important in larger and stronger infants.
- Palpate the spinous process that is even with the iliac crests. This is L4. Locate one interspace above (L3-L4) or one space below (L4-L5) as the site.
- Glove.
- Prep 3 times with alcohol and sponges from the LP tray. Drape with the drape in the tray or with a Steridrape R.
- Use 22-gauge short spinal needle from kit or one with a clear hub from the cupboard. Hold needle/stylet with both hands, thumb on the hub and first fingers guiding into the interspace, aiming for the umbilicus. Insert slowly. One may not feel "pop" as is common with the larger children. Remove stylet and check for CSF. Return stylet and advance if no return. One may need to rotate the needle slightly to increase or initiate the flow. If unsuccessful repeat attempt in next interspace up or down, but never go above the L2-L3 interspace.
- When collecting for diagnostic purposes, the spinal fluid should be collected in the tubes from the kit in the following order; Tube 1 - culture and STAT gram stain, Tube 2 - glucose and protein, Tube 3 - cell count.
- The stylet should be replaced and the needle removed, covering the site with a 2 x 2 gauze until a bandage can be placed.
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
Adequate quantities of serum may be obtained via heel stick in almost any neonate. If done properly, hemolysis should not be a significant problem. The skin's blood supply is located at the junction of the dermis and subcutaneous tissue, 0.35 to 1.6 mm from the skin surface.
Prewarming with the commercially-available heel warmers or with a diaper which has been warmed under a warm faucet and taped around the heel often increases the blood supply and arterializes the sample. The area should be cleaned thoroughly with alcohol swab. The person performing the procedure should wear appropriately fitting gloves.
The heel puncture should be done on the most medial or lateral portions of the plantar surface of the heel, not on the posterior curvature, to avoid the calcaneous. The lancets are designed to enter no deeper than 2-3 mm. If using a scalpel blade, the blade should enter the skin no more than 2-3 mm. After the puncture, wipe the first small drop off to rid the skin of the tissue juices that may increase clotting at the site.
Hold the ankle area with the 3 fingers on your ulnar side while placing your thumb behind the heel and your second finger just below the ventral surface of the toes. By alternately pressing the lateral three fingers , followed by a milking motion of the second finger, blood can be expressed. The fingers should be relaxed for a few seconds periodically to allow refilling. To prevent bruising, caution should be used to limit squeezing with the finger tips. To prevent hemolysis, allow large droplets to form, collecting the drops as they form into the microtube, not scraping the blood into the tube.
Fingerstick sampling is used for capillary blood gas analysis in our NICU and may be used for additional laboratories as well. The technique is similar to heelstick in that only the medial and lateral aspects of the finger are stuck. The milking motion includes the whole finger and even portions of the hand.
References
Blumenfeld, et al: Recommended site and depth of newborn heel skin punctures base on anatomical measurements and histopathology. Lancet 1979;1:230.
Iowa Neonatology Fellows and Neonatal Nurse Practitioners
Peer Review Status: Internally Peer Reviewed - 4/21/11
Background and general information
Percutaneously placed, central intravenous catheters (PICC) are an important part of neonatal patient management at the University of Iowa Stead Family Children's Hospital and elsewhere. They have proven valuable in helping to provide adequate long-term nutritional support as well as providing long-term vascular access for the administration of medications.
The risk/benefit ratio of placement and duration of central line use must always be considered on an individual basis.
Who needs a PICC line?
- Infants who need prolonged parenteral nutrition
- Infants needing long term intravenous drug therapy
- Infants needing hyperosmolar intravenous fluids or irritating medications
- Infants with difficult or limited intravenous access
Risks of PICC line placement and use
Generally the risks of percutaneously placed intravascular central catheters are lower than those of catheters placed surgically. Risks include but are not limited to:
- Catheter sepsis – Infection is the most common complication from the use of PICC lines. It is more common in the smallest and most premature infants at the time of insertion. Catheter insertion should be delayed, if possible, in infants with positive blood cultures until a negative culture is obtained. Catheters already in place in an infant who develops a blood stream infection should have their PICC line removed if the infection cannot be cleared. Removal of the line is considered for blood cultures growing S. aureus, gram negative organisms, or candida species.
- Phlebitis. Mechanical phlebitis may occur in the first few days after insertion and is often a normal response of the body to the irritation of the catheter. Mild phlebitis, (mild erythema and/or edema) can be managed with warm dry compress in infants with intact skin, and extremity elevation. If phlebitis is severe, (streak formation, palpable venous cord, and/or purulent drainage) or if there are signs of catheter related infection the line will likely need to be removed. Please discuss removal with supervising physician.
- Catheter migration or malposition and vessel erosion. This can occur during insertion or at anytime during the catheter dwell time. Consequences of malposition or erosion in the infant are dependant on the site of the tip location and can include: pericardial, pleural or peritoneal effusion, cardiac arrhythmias, tissue extravasation/infiltration or migration into small vessels.
Initial confirmation of PICC line tip location:
- Upper extremity vessel: Obtain an A/P chest X-ray with the arms in adduction and the head turned away from the side of placement. Successful central placement in the superior vena cava, (SVC) is above the pericardial reflection line. If the tip cannot be clearly visualized a right posterior oblique film, 20 degrees off midline) should be taken.
- Lower extremity vessel: Obtain two X-ray views of the abdomen, one A/P and one cross table lateral. Successful central tip placement is in the inferior vena cava, (IVC)
- A neonatologist and nurse practitioner, or at least 2 medical team members should evaluate the X-rays to confirmed line placement. Location of the catheter tip must be documented in the procedure note.
Monitoring PICC line tip location during line maintenance:
- Weekly and as needed monitoring of PICC lines with appropriate X-ray studies with discussion of current tip location in rounds and documentation when appropriate in the progress note.
- Documentation of tip location when seen on routine X-rays in progress notes.
Catheter dysfunction:
- Characterized by inability to infuse fluids, withdraw blood or the leaking of fluid.
- May be caused by malposition, thrombosis, presence of precipitates, lipid deposits, or mechanical issues, (e.g., patient positioning, dressing too tight).
- If cause cannot be resolved the line should be removed after discussion with NNP, Neonatal Fellow or Staff.
Catheter Breakage:
- Can be severed by the introducer needle during insertion, snap because of excessive tension or rupture because of excessive pressure. The intravascular portion of the catheter is at risk of embolization.
- In the event of breakage; grasp and secure the exposed portion of the catheter to prevent migration. If there is no exposed portion apply pressure over the venous tract above the insertion site, immobilize the infant and obtain an X-ray. Staff Neonatologist and Pediatric Surgery should be notified for intervention.
- Damaged or broken catheters must be removed and replaced. Repaired catheters and replaced catheters over a guidewire place the patient at increased risk for infection and embolization. If no other options are available a repaired catheter should be considered temporary.
- Prophylactic antibiotic coverage should be considered.
Tethered Catheter:
- Difficulty removing a catheter may be due to formation of a fibrin sheath or secondary to sepsis.
- Limit the number of attempts to remove the catheter by experienced personnel to two.
- Reposition the limb to minimize bends in the catheter and slowly remove the catheter.
- Inject saline into the catheter while slowly removing the catheter.
- Application of warm, dry heat over the catheter tract and insertion site for 20 minutes may decrease encountered resistance due to venospasm. Avoid direct pressure on the insertion site and catheter tract during removal to help avoid venospasm.
- Notify surgery and the attending Neonatologist for all incidents that involve catheters that have a history of dysfunction and can't be removed by the usual traction force.
- Inform the family that the PICC line may be required to be surgically removed
Catheter placement procedure
- PICC line catheters in the NICU are placed by trained professionals who meet ongoing competency requirements. In our NICU these professionals include Neonatologists, Neonatology Fellows and Nurse Practitioners.
- Parents are informed about the needs, risks and alternatives for the procedure.
- PICC line appropriate fluids are ordered and are on the unit
- A time out procedure form is completed.
- Consideration is given to pain control options appropriate for the infant.
- An introducer and catheter kit are obtained from the Omnicell under the patient's name. Unused kits and introducers are replaced in the Omnicell and credited under the patient's name.
- A PICC line cart with additional line placement and dressing supplies is brought to the patient room for easy access.
- Hand washing and sanitizing is completed.
- Vein selection typically involves the basilic, cephalic, saphenous, popliteal, external jugular, temporal and post auricular veins. Catheterization success is highest in veins that have not previously been used for peripheral IV's. The length of catheter needed is measured on the infant with a paper tape measure in centimeters prior to scrubbing the site and donning sterile attire.
- Maintain sterile technique. Everyone wears a hat and mask. The LIP/Physician and assistant wear sterile gowns and gloves in addition to hats and masks with eye shield recommended for the inserter.
- Catheters are cut prior to insertion to the proper length to minimize problems associated with migration. The guillotine razor found in the catheter kit is used to cut the catheter.
- Follow manufacturer's instructions for insertion and use Betadine scrub if infant is less than 2 months of age and 2% chlorhexidine gluconate for infants greater than 2 months. A large body drape is used to maintain sterile technique. Dress site according to unit recommendations. Run normal saline through line at 2 ml per hour until central tip placement is confirmed.
- Confirm central placement with appropriate X-rays and have second member of the medical team confirm. Inform nursing staff the line can be used as planned and complete the "blue card" Keep me Safe! I Have a Central Line."
- Complete procedure note. A note should be completed even if the attempt was unsuccessful.
- Complete charge form regardless if the procedure was successful or not.
Care and use of percutaneous central catheters
- Dressings should remain intact at all times after line placement. Dressings should be changed by a trained team member from the nursing/ARNP/physician staff if non-secure or sterility is questioned.
- IV fluids infusing in a PICC line should contain heparin usually at a concentration of 0.25 units per ml of fluid if the rate is greater than or equal to 2ml per hour and 0.5 units per ml if the rate is less than 2ml per hour. Total heparin received by an infant in 24 hours should not exceed 100units/kg/day.
- Maximum fluid rates for each type of catheter are recommended by the manufacturer and should be considered before line placement.
- When a PICC line is used for nutritional purposes, glucose concentrations up to 25% may be used to provide adequate calories if the catheter has been successfully placed in the vena cava. However, in doing so one should try to use less concentrated dextrose solutions since the risk of thrombosis goes up with the use of increasingly hyperosmolar solutions. Attempts should be made to fully utilize other less hyperosmolar means of providing calories. This might include using lipid solution to provide additional calories and/or to use a faster rate of infusion with a less concentrated dextrose solution. These considerations should be evaluated on a continuing basis. When central placement is not achieved in the vena cava dextrose concentration should be kept at 12.5% or less.
- When PICC lines are used for medications careful attention must be paid to fluid compatibilities. To prevent contamination of the line enter the line only when absolutely necessary and maintain sterility.
- PICC lines should not be used for routine lab draws or to give a red blood cell transfusion due to increased risk of infection, clotting of the line and hemolysis of red blood cells.
- A notation of PICC line tip location should be documented in the daily note if visible on X-ray.
- A PICC line should be removed as soon as it is safe to do so. If total fluid intake is 100ml/kg/day by any other route and tolerated, and if the line is no longer needed for medication administration the line can be removed.
- A nurse practitioner or a physician may remove a PICC line. A Central Line Removal procedure note must be completed. At the time of its removal, the length of the catheter from its tip to entry point into the plastic hub should be measured and compared to the placement procedure note and recorded in the removal procedure note. Discrepancies should be discussed with the supervising physician if breakage is a concern.
- Always consider the PICC line as a source of infection or complication with any clinical deterioration in the infant.
- If repair of a PICC line is considered and possible it should be discussed with the supervising physician and performed under sterile technique according to the manufacturer's recommendations
References
- Coit AK, Kamitsuka MD; Pediatrix Medical Group. Peripherally inserted central catheter using the saphenous vein: importance of two-view radiographs to determine the tip location. J Perinatol. 2005 Oct;25(10):674-6
- Harako ME, Nguyen TH, AJ Cohen. Optimizing the patient positioning for PICC line tip determination. Emerg Radiol 2004;10:186-189.
- Hogan MJ. Neonatal vascular catheters and their complications. Radiol Clin North Am 1999;37:1109-1125.
- MacDonald, MG, Ramasethu, J. editors. 2002. Atlas of Procedures in Neonatology
- Nadroo AM, Lin J, Green RS, Magid MS, IR Holzman. Death as a complication of peripherally inserted central catheters in neonates. J Pediatr 2001;138:599-601.
- Pettit J, M Mason-Wyckoff. 2001 Peripherally Inserted Central Catheters: Guideline for Practice. National Association of Neonatal Nurses.
- Standards of Practice-Department of Nursing, University of Iowa Hospital and Clinics, Children's and Women's Services, Policies and Procedures. N-CWS-PEDS-08.055. Central Catheters-Pediatric Bundle. Also 08.060, 08.130, 08-140. N-07.050 and IC-02.000.
Reviewed 4/2011. A. Gronstal ARNP, M. Lofgren ARNP, J. Klein MD, J. Dagle MD
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
Indications.
- To clear airways of secretions.
- To keep artificial airway patent.
- To obtain material for analysis of culture.
- In-line suctioning preferred for indications other than obtaining material for culture.
Pre-assemble suction equipment. Recommended suction catheters are 5 or 6 French for 2.5 mm ET tube, 6 French for 3.0 ET tube and 8 French for 4.0 ET tube. The amount of suction applied to the catheter should be between 40-80 mmHg.
Suction between feedings or discontinue feedings for period of treatment.
Auscultate chest prior to suctioning. Oxygenation prior to suctioning will be done with an FiO2 no greater than 0.10 above that being used to ventilate the infant. Monitor heart rate continuously. Suction should not be applied while the catheter is being inserted down the ET tube. The tip of the suction catheter will not be inserted beyond the end of the tube. When withdrawing the catheter, continuous suction is applies. The procedure should not take longer than 10 seconds. Following suctioning, ventilate the infant with an FiO2 no greater than 0.10 above that used prior to suctioning. The PaO2 should be raised to a level comparable to that prior to suctioning.
Do not add saline unless necessary. Saline may be used if the infant has thick tenacious secretions which cannot be extracted by using suctioning alone. Normal saline for secretions for Respiratory Therapy use is instilled into ET tube and 3-5 ventilated breaths performed prior to suctioning as above.
Vibration and percussion (CPT) will not be performed routinely prior to suctioning. If the need for CPT is documented, it must be ordered by a physician describing the area to be treated and the frequency of treatments.
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
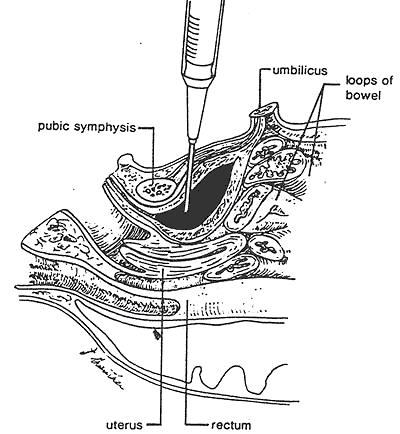
Indications: bladder aspiration is performed to obtain sterile urine for culture. A suprapubic bladder tap is not necessary for Group B strep latex antigen studies (i.e., a bag specimen is adequate).
Be certain that voiding has not occurred within the previous hour so that the bladder has an adequate amount of urine. The infant is restrained in the frog leg position. The pubic area is prepped 3 times with an alcohol swab. A 25-gauge needle attached to a 3-ml syringe is directed perpendicularly to the skin just superior (0.5 cm) to the symphysis in midline and advanced to its hub. Full-term infants sometimes require a 22-gauge needle (which need not be inserted to the hub). The needle is withdrawn, slowly, with slight pressure pulling back on the syringe.
A minimal amount of hematuria may be seen after an attempt, but otherwise the risks are minimal. Rare complications include bladder wall hematoma, lacerated vessel on anterior bladder wall, perforation of hollow viscus, osteomyelitis of pubic bone or abdominal wall abscess.
If no urine is obtained, the infant should have a U-BagR placed with repeat attempt in 1 hour. If unable to obtain a specimen, a catheterized specimen may need to be obtained but this procedure is more difficult to perform and may be riskier.
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
Pulmonary air leak is an anticipated risk of mechanical ventilation. Drainage of air or fluid accumulation in the thorax is an important and necessary skill and is often performed emergently.
Indications
- Evacuation of pneumothorax
- Evacuation of large pleural fluid collections
- chylothorax
- empyema
- hemothorax
- A small spontaneous pneumothorax in the absence of lung disease will most likely resolve without intervention.
When evaluating a suspected pneumothorax, auscultation and transillumination of the chest should be performed. Note that false positives may result from subcutaneous edema or air. If positive, consider needle aspiration performed with a 20 or 22 gauge needle connected to a 30 cc syringe via a 3-way stopcock. After prepping with alcohol, insert needle 3-5 mm into the chest wall in the fourth or fifth intercostal space in the anterior axillary line. If the infant is supine, air may be easier to access via the second intercostal space in the mid-clavicular line.
If pneumothorax is under tension or reaccumulates following needle aspiration, the insertion of a chest tube (CT) will be necessary. Appropriate insertion sites include the fourth, fifth or sixth intercostal spaces in the anterior axillary line. The nipple is a landmark for the fourth intercostal space.
Insertion (see figure below)
- A 8, 10 or 12 French CT used depending on the size of the infant.
- Position infant supine or with the affected side elevated 45-60 degrees off the bed using a towel or blanket as back support. This has an advantage of allowing air to rise to the point of entry and of encouraging the correct anterior direction of the CT.
- The skin is prepped with alcohol and sterilely draped.
- A 1 cm incision is made through the skin on top of the rib to facilitate entry of the CT. Using a small curved forceps, separate the tissue down to the pleura.
- Grasping the end of the CT with the tips of curved forceps, apply pressure until the pleural space is entered. Do not use the trocar. Direct CT toward apex of thorax (midclavicle) and advance CT assuring that side holes are within thorax. Observe for cloudiness, vapor or bubbling in CT to verify intrapleural location.
- The chest tube should be inserted 2-3 cm for a small preterm infant and 3-4 cm for a term infant. (These are approximate guidelines only.)
After CT insertion connect the tube's distal end to a water seal system such as a PleurevacR. To apply suction, use 15-20 cm of water in the PleurevacR column. If multiple CTs are placed, each CT should be connected to it's own water seal system and suction source.
Secure CT to skin with suture and cover incision site with vaseline gauze and/or TegadermR dressing.
After thoracentesis or CT insertion a chest x-ray, A/P and lateral should be obtained.
If there is a persistent pneumothorax despite a properly placed CT, consider increasing the column of water by 5 cm increments up to 30 cm before inserting a second CT.
Prior to removal, the CT should be clamped for 2-4 hours or longer. If there is no reaccumulation of air, the CT can be removed.
Complications
- Misdiagnosis with inappropriate CT placement
- Malpositioned CT
- Trauma
- lung laceration or perforation
- laceration and hemorrhage of major vessel (axillary, intercostal, pulmonary, internal mammary)
- puncture of viscus with path of tube
- Infection
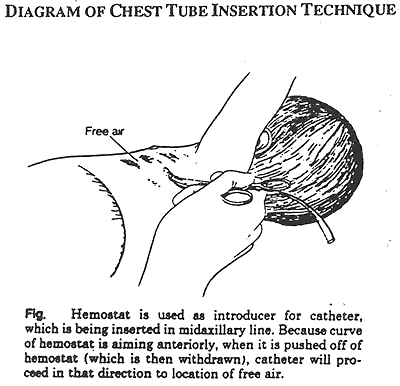
Reference
Mehrabani D, Kopelman AE: Chest tube insertion: A simplified technique. Pediatr 1989;83:784-785.
Iowa Neonatology Fellows
Peer Review Status: Internally Peer Reviewed
Pneumopericardium generally occurs in infants receiving assisted ventilation or vigorous resuscitation. Pneumopericardium becomes clinically significant when the pericardial air is under enough pressure to impede cardiac output and tamponade the heart.
Indications
- Pneumopericardium with tamponade.
- Pericardial effusion with tamponade.
Evaluation
Physical examination of an infant with suspected pneumopericardium will reveal tachycardia or bradycardia, muffled or distant heart sounds and decreased blood pressure. Chest x-ray, time permitting, will demonstrate air encircling the heart on both the anterior-posterior and lateral views. The volume of pericardial air seen on x-ray may not correlate with the clinical signs of circulatory compromise. Transillumination is sometimes positive, but pneumopericardium may be confused with pneumothorax or pneumomediastinum.
Technique
- Cleanse skin over xiphoid, precordium, and upper abdomen with alcohol.
- Use (1 1/2 inch) 16, 18 or 20 gauge angiocath attached to a 3-way stopcock and 30 cc syringe. If clinical situation permits, consider cutting 1 or 2 small (1 mm) holes (to function as sideports) near end of catheter using blade.
- Insert the catheter 0.5 cm to the left of and just below the infant's xiphoid, directing it toward left shoulder, aspirating with the syringe as the catheter is advanced. When the pericardial space is entered and air is obtained, remove stylet. Aspiration of air usually results in immediate improvement in hemodynamic status (see figure).
If air reaccumulates, secure catheter in place and attach to continuous suction via water seal system using 5-10 cm of water in the column.
Confirm catheter position by chest x-ray.
Potential complications include myocardial puncture or irritation, hepatic laceration, injury to major vessel, pneumothorax, hemothorax, and infection.
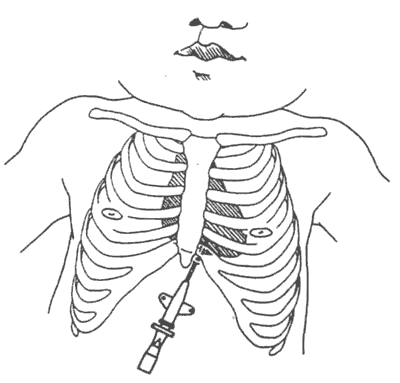
Reference
Mehrabani D, Kopelman AE: Chest tube insertion: A simplified technique. Pediatr 1989;83:784-785.
Iowa Neonatology Fellows
Revised: John Dagle MD, PhD
Peer Review Status: Internally Peer Reviewed
Indications
- Provide airway for mechanical ventilatory support.
- Administration of surfactants or other medications directly into the lungs.
- Relieve critical upper airway obstruction.
- Provide route for selective bronchial ventilation.
- Assist in pulmonary hygiene when secretions cannot be otherwise cleared.
- Obtain direct tracheal cultures.
The correct endotracheal tube (ETT) size and length of insertion (tip to lip distance) can be estimated from the infant's weight.
| Weight | ETT | Depth of Insertion (cm) |
|---|---|---|
| 1 kg | 2.5 | 7 |
| 2 kg | 3.0 | 8 |
| 3 kg | 3.5 | 9 |
| 4 kg | 4.0 | Add 1 cm for each additional kg of body weight. |
| Insertion Depth (cm) = 6 + wt (kg) | ||
The tube should not fit tightly between the vocal cords in order to minimize upper airway trauma.
In most cases an infant can be adequately ventilated by bag and mask so that endotracheal intubation can be done as a controlled procedure. The ONE IMPORTANT EXCEPTION is in cases of known or suspected congenital diaphragmatic hernia.
Preparation is important to performing successfully. Check availability of following equipment prior to procedure - suction, laryngoscope with functioning light source, appropriate laryngoscope blade size (Miller 0 or Miller 1), supply of ETTs, CO2 detector, stethoscope, tape and adhesive. Premedication of the infant (sedation and analgesia) should be considered in all cases of elective intubations.
Technique
- Prior to attempting the insertion of an ETT and as indicated by clinical condition, one should ventilate the infant with bag and mask using 80-100% oxygen. If unable to insert the ETT within 30 seconds, ventilate the infant again for 30-60 seconds before reattempting intubation.
- Infant's head should be slightly extended (in the sniffing position) with the body aligned straight.
- The laryngoscope is held with the left hand. Pushing down gently on the larynx with the fifth finger of the left hand (or having an assistant do it) to provide slight cricoid pressure may help to visualize the vocal cords. Avoid extreme tension or tilt of the laryngoscope.
- The ETT is held in the right hand and inserted between the vocal cords so that the tip is 1-2 cm below the vocal cords.
- Ensure endotracheal position by the use of a CO2 detector- this has become a standard of care. The detector should change color (purple to yellow) by 5-6 breaths.
- Check tube position by auscultation of the chest (and abdomen) to ensure equal aeration of both lungs and observation of chest movement with positive pressure inflation.
- Secure ETT with two pieces of 1/4 inch adhesive tape placed on lip and securely around ETT.
- Verify the position of the ETT by chest x-ray.
Whenever a stylet is used for intubation, be sure that the stylet tip does NOT extend beyond the end of the ETT.
If the infant will require intubation for greater than 10- 14 days, consider the use of a palate plate to prevent formation of a palatal groove. Palate plates can be obtained by requesting a consultation from Pediatric Dentistry.
blackburnboyabseut.blogspot.com
Source: https://uihc.org/childrens/educational-resources/procedures-nicu-handbook
0 Response to "5fr Feeding Tube as Umbilical Catheter"
Post a Comment